Combustion Basics
Introduction to fundamental principles of combustion within Fired Heaters
This article will cover basic combustion principles relating to gaseous fuel burners inside Fired Heaters and Furnaces.
The term 'Combustion' is considered as the controlled release of heat energy from the chemical reaction between a fuel and an oxidizer.
The following is the chemical equation for the combustion of Hydrogen with Oxygen:
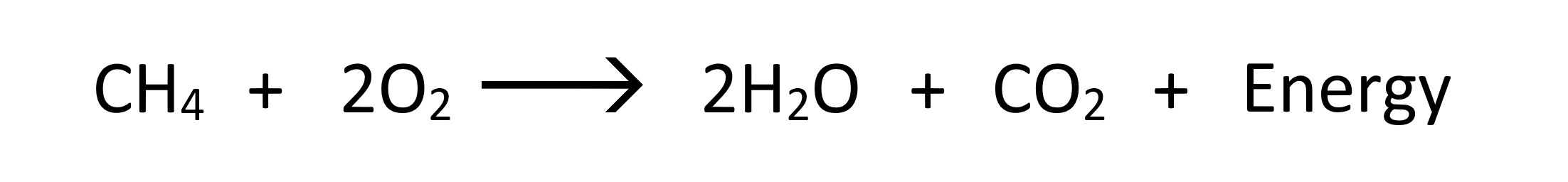
Methane Combustion Chemical Equations
Excess Air
This combustion equation above represents stoichiometric combustion of methane, where the exact amount of oxygen is utilised to burn methane and produce Carbon Dioxide and Water vapor.
Of course different hydrocarbon fuels require different amounts of oxygen. For example, Ethane requires 3.5 times moles of Oxygen, whilst Propane requires 5 times moles of Oxygen for balanced stoichiometric combustion.
However, in reality, exact stoichiometric conditions are not maintained within Fired Heaters or Furnaces. Instead, Fired Heaters are usually operated with excessive air conditions. This excess amount, is termed 'Excess Air', and is typically between 10-25% of the calculated stoichiometric theoretical value.
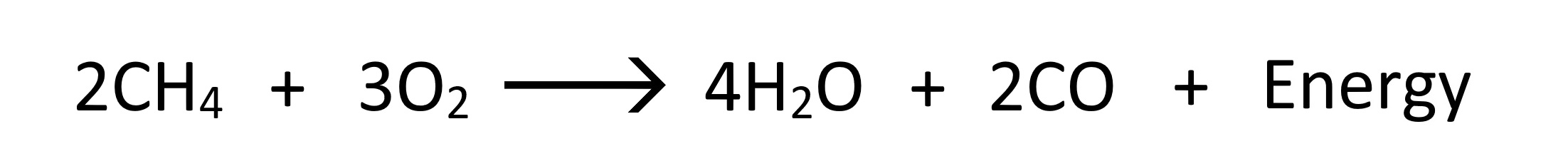
Carbon MonoxideIn conditions whereby insufficient oxygen is provided to the Fired Heater for burning the fuel, Carbon Monoxide is produced instead of Carbon Dioxide:
Methane Sub-stoichiometric Combustion - Producing CO
Other Combustion Products in Fired Heaters
There are unwanted reactions that occur during the combustion process resulting in “unintended” products of combustion.
These unwanted products are small in the overall percentage of the products of combustion, but have large operational and environmental impacts.
Refineries and Petrochemical facilities are usually required to operate Fired Heaters and Furnaces in accordance with strict Government regulations relating to the emission of these unwanted combustion products.
Nitrogen Oxides (NOx)
Nitrogen Oxides, or NOx for short, are probably the most notorious as they lead to the production of smog and acid rain. A detailed discussion of NOx formation is beyond the scope of this post, but the basics are that NOx is formed via three different mechanisms that occur during combustion:
- Thermal NOx: Generated in the core of the flame due to extremely high temperatures which can exceed 1000 C
- Prompt NOx: Generated by the quick reaction between nitrogen, oxygen, and hydrocarbon radicals
- Fuel NOx: Generated when fuel containing chemically bound nitrogen is burned. (Example: Ammonia, NH3)
Sulphur Oxides (SOx)If the fuel contains Sulphur, this can also react with Oxygen to form Sulphur Oxides, which are also harmful to the environment.
SOx is also corrosive and therefor Fired Heater and Furnace manufacturers are required to ensure that the materials utilised can tolerate such conditions.